An international team of scientists led by the University of Liverpool has produced a ‘structural movie’ revealing the step-by-step creation of an important naturally occurring chemical in the body that plays a role in some cancers.
S-Adenosylmethionine (SAMe) is a major methyl donor that is produced by the highly conserved Methionine Adenosyltransferase (MAT) family of enzymes. Methylation is an underpinning process of life and provides control for biological processes such as DNA synthesis, cell growth and apoptosis.
Tight regulation of the level of SAMe is essential for maintaining a healthy cell and dysregulation of SAMe is considered important in many diseases including liver and colon cancer.
X-ray crystallography
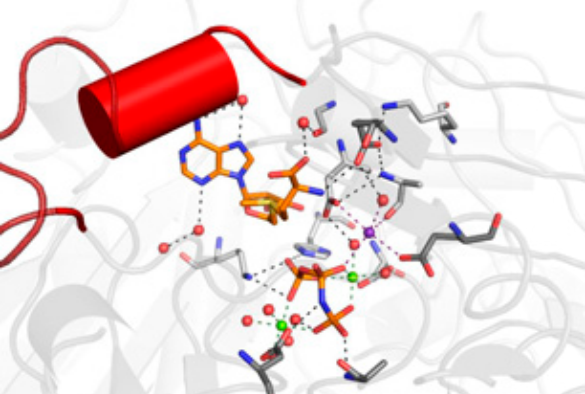
The international team, which includes researchers from the Center for Cooperative Research in Biosciences, Spain, and Cedars-Sinai Medical Center, Los Angeles, used X-ray crystallography to successfully unravel how the catalytic subunit MATα2 synthesises SAMe, with details of every atom’s location and behaviour as the synthesis takes place.
The work was led by Professor Samar Hasnain and Dr Svetlana Antonyuk who are co-directors of the University’s Barkla X-ray Laboratory of Biophysics and utilised some of Europe’s most powerful X-ray synchrotron sources, including ALBA in Spain and DIAMOND in the UK.
New therapeutic targets
Professor Samar Hasnain, said: “Though the relationship between SAMe and tumour growth has been known for some time, this molecule also has other important functions inside the cell that cannot be altered and there is currently no way of acting against it without affecting these other life-sustaining functions.
“The good news is that MATα2 is only overexpressed in adults with tumours therefore representing an excellent therapeutic target, which could open the door to the creation of highly targeted drugs that act exclusively on this enzyme rather than attacking other regions of the body.“
Dr Antonyuk, added: “Our work has provided a detailed insight into the synthesis of SAMe by this human enzyme and has opened the way to target it for therapeutic purposes for hepatocellular carcinoma and colon cancer.”
‘Crystallography captures catalytic steps in human methionine adenosyltransferase enzymes’ is published in the latest issue of the Proceedings of the National Academy of Sciences.