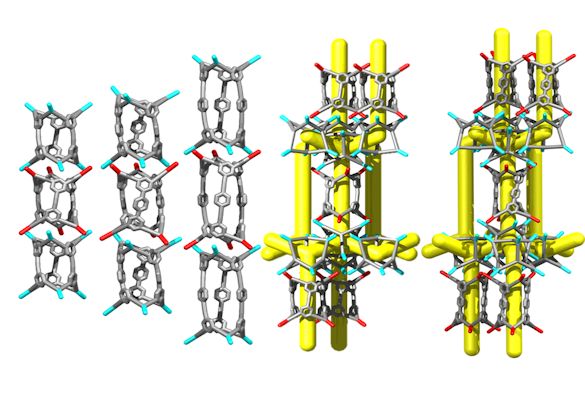
Image: Three 1-D nanotubes and two 3-D structures made from porous organic cages
Scientists at the University of Liverpool have developed a blueprint for controlling the packing of porous molecules into pre-designed structures, such as 1-D nanotubes and 3-D networks.
Such control over the solid state structure of materials has a profound effect on their properties, and could open up possibilities for better superconductors, optoelectronic materials, or organic photocatalysts.
The study, underpinned by calculations that rationalize the structures formed, is published in Nature Chemistry.
The porous molecules used in the study are examples of ‘porous organic cages’ (POCs). POCs have an internal cavity into which other smaller molecules can be loaded, such as water or carbon dioxide. When the cages form solid materials, they can arrange to form channels in which the small ‘guest’ molecules can travel from one cage to another.
The cage molecules are also soluble in common solvents, meaning they are easy to process, unlike most other porous materials such as metal-organic frameworks (MOFs).
However, until this study, it has been much harder to control the arrangements of POCs than the solid state structure of MOFs.
Liverpool Chemist and lead author of the study, Dr Anna Slater, said: “To control crystallisation, you need strong, directional interactions, and building blocks that are the right shape. We designed a new family of POCs to fit this brief, with the intention of forming nanotube structures.”
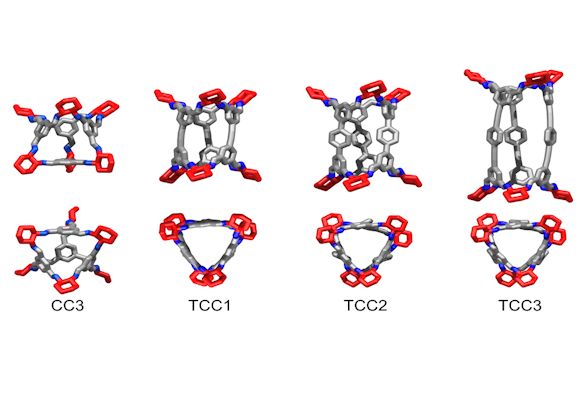
Most of the POCs made to date at Liverpool are tetrahedral in shape, and can be thought of as 4-way linker ‘building blocks’.
In this study, linear, 2-way linker POCs were made; these are capable of linking together using chiral recognition to form 1-D structures, or linking with 4-way linker cages to form more diverse solid-state structures.
One of these structures is one of the most porous molecular solids known (BET surface area = 2071 m2 g-1). This ‘mix and match’ cage pairing strategy parallels the development of isorecticular MOFs, where organic linkers are paired with metallic secondary building units to create designable, regular structures.
Dr Slater added: “With more building-blocks becoming available, high-throughput screening methods using this strategy are likely to yield a rich diversity of new materials with useful functions”.
This research also involved the University of Southampton and Imperial College London. It was funded by the Engineering and Physical Sciences Research Council (EPSRC) and the European Research Council.
Dr Slater will start a Royal Society-EPSRC Dorothy Hodgkin Fellowship in December exploring the use of flow chemistry to discover, optimise, and scale up new materials. She has also recently been appointed as a proleptic lecturer in the University’s Materials Innovation Factory (MIF).
Due to open in 2017, the £68M facility will revolutionise materials chemistry research and development through facilitating the discovery of new materials which have the potential to save energy and natural resources, improve health or transform a variety of manufacturing processes.
Image below: The POCs used as building blocks in the study: side view (top) and top view (bottom)