Credit: Louise Thompson
Louise Thompson is a PhD student in the Department of Molecular and Cellular Physiology.
Most of the research behind new medical advances is carried out using either animal tissues or cancer cells. Both tools have their problems: results from animals and humans do not always match up and cancer cells grown for years in laboratories often do not mimic the tissues they originally came from very well. Bridging the gap between whole animals and simple cells can be a challenge during the development of new treatments, but this is beginning to change since scientists have learned how to grow organoids.
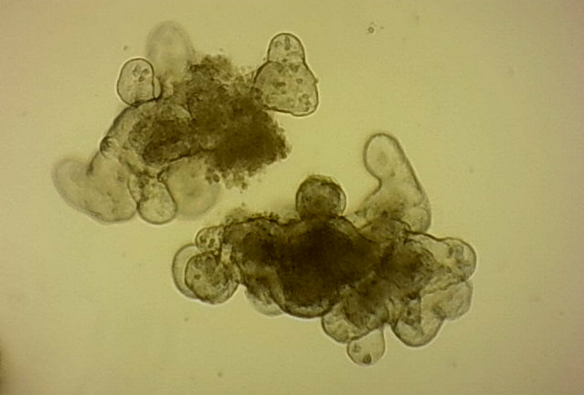
Organoids are clusters of cells that organise themselves into mini versions of our organs. They are grown from stem cells, which has only become possible with the discovery of the precise conditions needed to keep stem cells alive outside the body. Organoids were first made from intestines but have since been made for many other tissues, including liver, breast and even brain cells. This will allow scientists to better study the development and diseases of these organs.
They are grown in a gel that allows them to develop three-dimensionally, so they mimic the architecture of our organs much more realistically than a simple layer of cells. Stem cells from the intestine multiply to form a ball, in which the hollow centre is like the space inside the intestine. The surface of these balls then buds outwards at various points to form pocket-like extensions. This is similar to the intensely folded surface of the gut wall.
Organoids have several advantages over existing approaches. Stem cells are taken from animals or patients and continually multiply so the organoids can be maintained for months. They provide an unlimited supply of material for study, meaning fewer animal studies are required. Making organoids from patients also raises intriguing possibilities for personalised medicine.
In traditional cell cultures every cell is identical but stem cells can form many different cell types so organoids contain a much more realistic mixture of cells. For example, M cells are specialised cells in the gut wall that act as surveillance posts, capturing bacteria from the gut and showing them to the immune system so it can monitor for danger. Some harmful bacteria exploit this to invade the gut wall. It was previously tricky to grow M cells in the lab for study, but they can be grown in organoids. When added to organoids, Salmonella, a bacterium that causes food poisoning, infected M cells more often than other cell types, suggesting this may be a route of infection in humans.
Some common disease-causing bacteria are surprisingly difficult to grow in the lab, making them hard to study. Clostridium difficile causes numerous cases of diarrhoea every year, a serious condition in frail patients. It has been difficult to grow C. difficile because it requires conditions without oxygen, but researchers in the US have shown that the bug can survive inside intestinal organoids. Bacteria were injected into the centre of intestinal organoids and produced a toxin that made the organoid wall leakier, damaging its ability to act as a barrier.
Organoids made from patient biopsies are allowing us to investigate differences between individuals. Patients with cystic fibrosis show varied responses to treatments. One group of researchers grew organoids from patient biopsies and tested their response to different combinations of drugs. In the future this may be used to quickly find the best treatment for each individual.
Tumours also vary hugely between individuals. Dutch researchers grew organoids from patients with colorectal tumours and identified genetic changes that had occurred in the tumour cells compared to the patient’s healthy tissue. They were then able to see how these altered the way the cells behaved. They tested anti-cancer drugs on the organoids and could tell which drugs did and did not kill the tumour cells.
Imagine if organoids were routinely made from tumour biopsies and used to identify the best chemotherapy combination for each patient. This is certainly plausible, but the process will first need to be made quicker and cheaper.
All this makes organoids an exciting new tool for researchers. Most work currently focuses on the stomach and intestine, but the technique is quickly expanding to other tissues such as liver, breast and brain. Organoids will transform the way we conduct medical research, from basic understanding to drug development and personalised therapies. Expect to hear much more about them in the future.
This article was originally published on The Conversation. Read the original article.